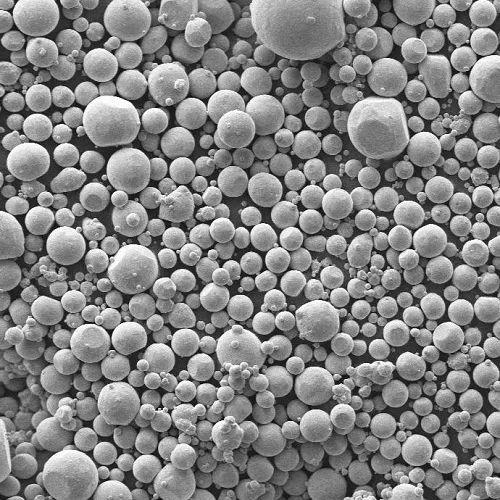
Magnesium oxide, commonly called magnesia, is a white solid mineral with the chemical formula MgO. It occurs naturally as periclase but is primarily produced industrially by calcining magnesium carbonate or magnesium hydroxide. This compound possesses an exceptionally high melting point of approximately 2852 degrees Celsius, making it invaluable as a refractory material in furnace linings and crucibles for high-temperature processes like steel production. Its refractory nature also lends itself to use in fireproofing building materials. Beyond construction, magnesium oxide serves critical roles in agriculture as a soil pH adjuster and magnesium supplement for crops. In medicine, it acts as a dietary supplement to address magnesium deficiency and as a common antacid and laxative, though its bioavailability is lower than some other magnesium salts. Environmental applications include its use in flue gas desulfurization to remove sulfur dioxide from power plant emissions. It also finds use as an insulator in electrical cables, in the manufacture of certain types of glass and ceramics, and as an additive in animal feed and some food products. While generally stable, magnesium oxide dust can be an irritant to eyes and lungs, requiring careful handling. Its versatility, stability, and high-temperature resistance ensure magnesium oxide remains a vital industrial and chemical compound across numerous sectors.
(magnesium iii oxide)
Inquiry us
if you want to want to know more, please feel free to contact us. (nanotrun@yahoo.com)